Optimization of Arsenic Adsorption onto Activated Carbon of Potato Peel Using Response Surface Methodology
Abstract
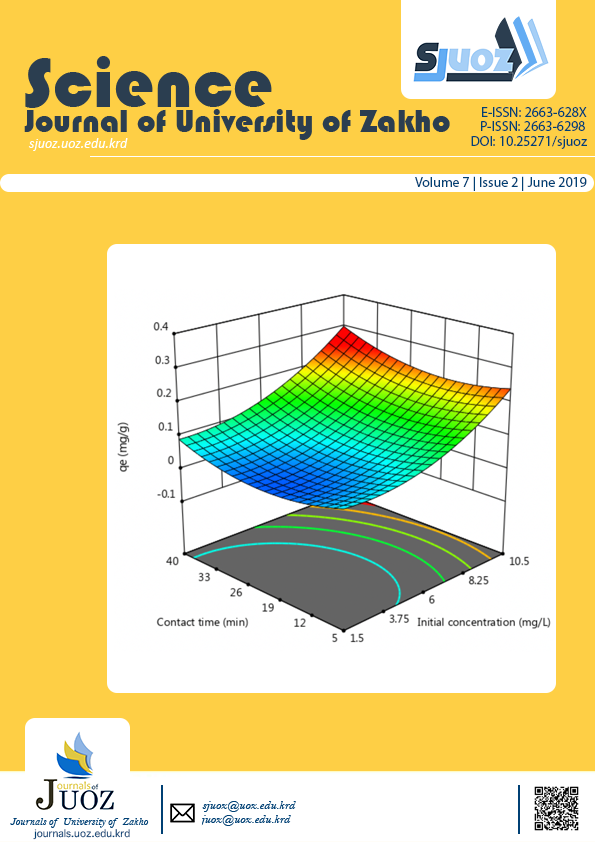
The present study was designed to optimize the adsorption of arsenic onto potato peel derived activated carbon (MPP-AC) by employing response surface method and central composite design. Adsorbent of cheap and locally available potato residue was produced based on chemical activation with H3PO4subsequently carbonization to produce the porous activated carbon. The individual and interactive effects of five variables including initial arsenic concentration, temperature, time, dosage amount and pH of the medium were investigated. Based on the statistic analysis (ANOVA), the quadratic model was developed associating the adsorption capacity (qe). The optimum conditions were obtained of 9.98 mg L-1of initial As (V) concentration, 28 °C of temperature, 39.7 min of time, 0.97 g of adsorbent dose and 7.3 of pH. The maximum adsorption capacity was 0.27 mg g-1and 76.5% removal efficiency. The equilibrium isotherms and kinetic studies for estimating the mechanism of process demonstrated a good fit to Langmuir model and the pseudo-second order, respectively. The results of this study showed that the feasibility of central composite design (CCD) to control adsorption process and indicated the use of activated carbon of potato residue have important implications for As (V) removal.
Full text article
References
Authors
Copyright (c) 2019 Akram A. Haji, Nidhal M.Sh. Mohammed

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License [CC BY-NC-SA 4.0] that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work, with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online.