One-Pot Synthesis, Pharmacological Evaluation, Docking Study, and DFT Calculations for Selected Imidazolidine-2,4-Diones
Abstract
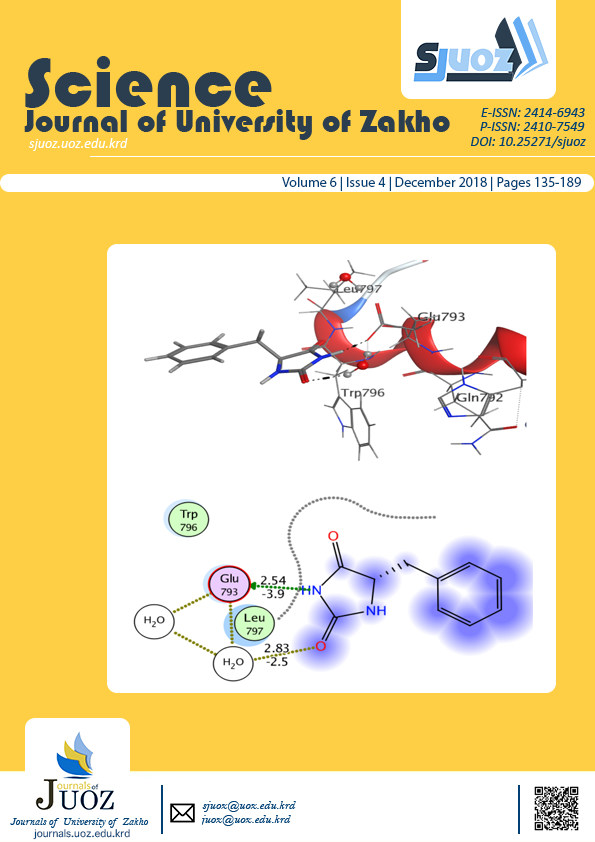
The title compounds with different 5-substituted imidazolidine-2,4-dione were synthesized through a solvent-free reaction. Imidazolidine-2,4-dione derivatives are found to be an active pharmacophore for design and development of various bioactive lead compounds. Positive values of energy obtained for compound 1and 3, while a negative value for compound 2 was calculated by DFT in Gaussian. keto-enol tautomerism was supported by energy values and indicated the most stable tautomeric form. The biological evaluation has been supported by docking studies using molecular operating environment program to show binding with androgen receptor.
Supplementary Materials: https://sjuoz.uoz.edu.krd/suppma
Full text article
References
Authors
Copyright (c) 2018 Zanko S. Jawhar, Hiwa O. Ahmad, Asmaa A. Haydar, Halala A. Abdullah, Sawza A. Mahamad

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License [CC BY-NC-SA 4.0] that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work, with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online.