The Effect of Deposition Time and Sulfurization Temperature on The Optical and Structural Properties of Iron Sulfide Thin Films Deposited from Acidic Chemical Baths
Abstract
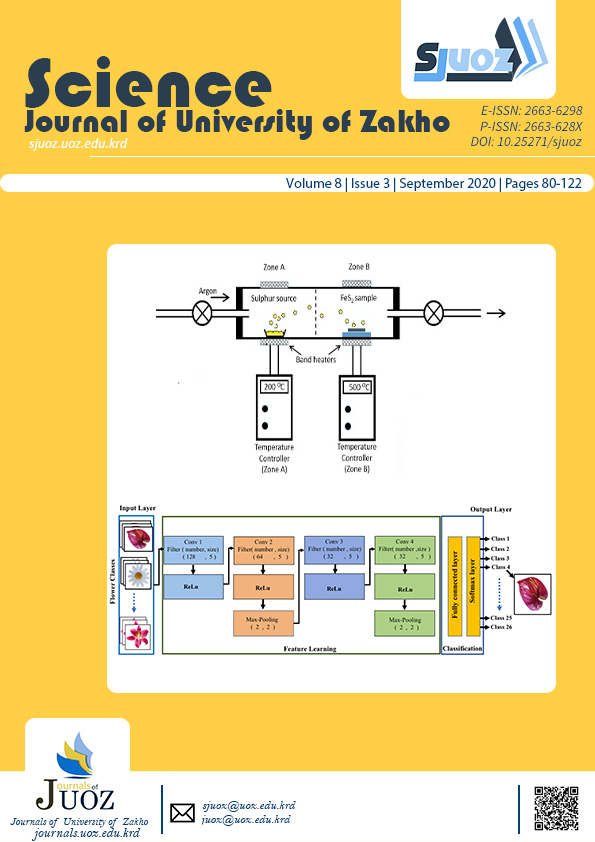
Pyrite phase FeS2 thin films have been grown by a two-stage process of chemical bath deposition followed by sulfurization. Thiourea and thioacetamide were used as sulfur precursors in separate baths. The deposition time was controlled for 1, 2, and 3 hours respectively. The as-deposited films were sulfurized at temperatures of 250 oC and 500 oC to form the pyrite phase. The effect of deposition time and sulfurization temperature on the structure, morphology and optical properties of the iron pyrite films obtained from the two separate baths were studied and compared. X-ray diffraction analyses established the formation of the pyrite phase in all the films after sulfurization, in addition to iron (II) oxide hydrate as impurities. All films showed further improvement in pyrite formation, crystallinity as well as an increase in crystallite size after sulfurizing at 500 oC. EDAX and SEM microscopy showed that the iron pyrite films produced from the bath containing thiourea, had better crystallinity and a higher iron content. The optical band gap of the iron pyrite films obtained with thiourea, was 2.1, 1.9 and 1.6 eV for the various deposition times. With thioacetamide, the band gap was 1.4 eV, for the deposition time of 3 hours.
Full text article
References
Authors
Copyright (c) 2020 Mark Paal; Isaac Nkrumah; Francis K. Ampong, David Ngbiche, Robert K. Nkum, Francis Boakye

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License [CC BY-NC-SA 4.0] that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work, with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online.