CARDIOVASCULAR BIOMARKERS, TYG INDEX AND TG/HDL-C RATIO IN SUBCLINICAL HYPOTHYROIDISM PATIENTS
Abstract
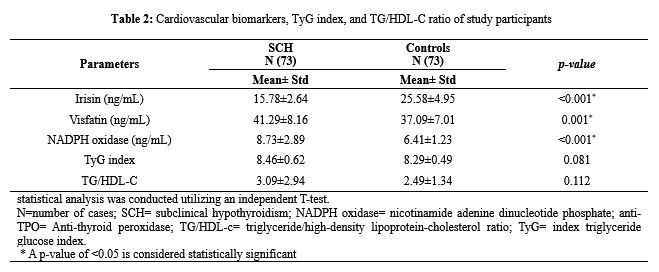
Subclinical hypothyroidism (SCH) is characterized by exhibiting a normal value of free thyroxine (T4) and excessive blood thyroid-stimulating hormone (TSH) concentration. SCH may be regarded as a prevalent concern of the emergence of overt hypothyroidism and cardiovascular disease (CVD). Disturbance in irisin, visfatin, NADPH oxidase, triglyceride/high-density lipoprotein-cholesterol (TG/HDL-c) ratio, and triglyceride glucose (TyG) index are associated with an increased cardiometabolic risk. The present study aimed to investigate serum levels of visfatin, irisin, NADPH oxidase (gp91phox), TyG index, TG/HDL-c ratio, and anti-TPO in patients with SCH in comparison to apparently healthy individuals. A study of the case-control method was carried out at Vin Specialist Laboratory, Kurdistan Region, Iraq, involving 146 subjects, including 73 newly diagnosed subclinical hypothyroid patients and 73 healthy controls. Biochemical tests such as glucose, TSH, FT4, FT3, Anti-Thyroid Peroxidase (anti-TPO), TG, and HDL-C were analyzed using Cobas6000 (Roche), whereas, enzyme-linked immunosorbent assay was conducted to estimate the value of serum visfatin, NADPH oxidase (gp91phox) and irisin. There was significantly a higher mean serum level of NADPH oxidase in 73 patients with SCH compared to that of 73 healthy individuals (8.73±2.89 ng/mL, 6.41±1.23 ng/mL, P=0.004). Mean serum levels of visfatin (41.29±8.16 ng/ml) and NADPH oxidase (8.73±2.89 ng/ml) were elevated in subclinical hypothyroid patients in comparison with healthy individuals with significant differences (p<0.001 and p=0.001), whereas, the mean serum level of irisin (15.78±2.64 ng/ml) was reduced in the SCH patients compared to healthy individuals (p=<0.001). The study found that, in contrast to healthy control individuals, patients with subclinical hypothyroidism had lower levels of serum irisin and higher mean values of TG/HDL-C, visfatin, TyG index, and NADPH oxidase
Full text article
References
Abdullah, n. M., gadallah, f. A., hamid, f. F. A., el-moneam, t. M. A., el-baki, r. S. A., & ibrahim, d. M. (2012). Serum visfatin in relation to some parameters of iron metabolism in egyptian subjects with altered glucose tolerance. Egyptian Journal of Biochemistry & Molecular Biology, 30(2). https://www.ajol.info/index.php/ejbmb/article/view/83461
Alhubaish, E. S., Alibrahim, N. T., Mansour, A. A., Alhubaish, E., & Alibrahim, N. (2023). The Clinical Implications of Anti-thyroid Peroxidase Antibodies in Graves’ Disease in Basrah. Cureus, 15(3). https://doi.org/10.7759/cureus.36778
Al-Mousawi, M., Salih, S., Ahmed, A., Abdullah, B., Ahmed, A. I., & Abdullah, B. I. (2024). Serum Vitamin B12 and Holotranscobalamin Levels in Subclinical Hypothyroid Patients in Relation to Thyroid-Stimulating Hormone (TSH) Levels and the Positivity of Anti-thyroid Peroxidase Antibodies: A Case-Control Study. Cureus, 16(6). https://doi.org/10.7759/cureus.61513
Alshaikh, E. M., Omar, U. M., Alsufiani, H. M., Mansouri, R. A., Tarbiah, N. I., Alshaikh, A. A., ... & Al Doghaither, H. A. (2019). The potential influence of hyperthyroidism on circulating adipokines chemerin, visfatin, and omentin. International journal of health sciences, 13(2), 44. https://pmc.ncbi.nlm.nih.gov/articles/PMC6436451/
Aroda, V. R., & Ratner, R. (2008). Approach to the patient with prediabetes. The Journal of Clinical Endocrinology & Metabolism, 93(9), 3259-3265. https://doi.org/10.1210/jc.2008-1091
Aronis, K. N., Moreno, M., Polyzos, S. A., Moreno-Navarrete, J. M., Ricart, W., Delgado, E., ... & Mantzoros, C. S. (2015). Circulating irisin levels and coronary heart disease: association with future acute coronary syndrome and major adverse cardiovascular events. International Journal of Obesity, 39(1), 156-161. https://doi.org/10.1038/ijo.2014.101
Bashir, H., Farooq, R., Bhat, M. H., & Majid, S. (2013). Increased prevalence of subclinical hypothyroidism in females in mountainous valley of Kashmir. Indian journal of endocrinology and metabolism, 17(2),276-80. https://doi.org/10.4103/2230-8210.109709
Bhardwaj, S., Bhattacharjee, J., Bhatnagar, M. K., Tyagi, S., & Delhi, N. (2013). Atherogenic index of plasma, castelli risk index and atherogenic coefficient-new parameters in assessing cardiovascular risk. Int J Pharm Biol Sci, 3(3), 359-64. https://www.ijpbs.com/ijpbsadmin/upload/ijpbs_526938e855804.pdf
Biondi, B., Cappola, A. R., & Cooper, D. S. (2019). Subclinical hypothyroidism: a review. Jama, 322(2), 153-160. https://doi.org/10.1001/jama.2019.9052
Bocale, R., Barini, A., D ‘Amore, A., Boscherin, M., Necozione, S., Barini, A., ... & Lombardi, C. P. (2021). Thyroid hormones modulate irisin concentrations in patients with recently onset hypothyroidism following total thyroidectomy. Journal of Endocrinological Investigation, 44, 1407-1412. https://doi.org/10.1007/s40618-020-01432-0
Choi, Y. M., Kim, M. K., Kwak, M. K., Kim, D., & Hong, E. G. (2021). Association between thyroid hormones and insulin resistance indices based on the Korean National Health and Nutrition Examination Survey. Scientific Reports, 11(1), 21738. https://doi.org/10.1038/s41598-021-01101-z
Closs, C., Vargas-Uricoechea, H., Schwarzstein, D., Lobo, M., Lagranja, E., Godinez-Leiva, E., & Nogueira, J. P. (2023). Relationship of subclinical hypothyroidism on epicardial adipose tissue: a systematic review and meta-analysis. Current Problems in Cardiology, 48(7), 101674. https://doi.org/10.1016/j.cpcardiol.2023.101674
Dakroub, A., Nasser, S. A., Kobeissy, F., Yassine, H. M., Orekhov, A., Sharifi‐Rad, J., ... & Eid, A. H. (2021). Visfatin: An emerging adipocytokine bridging the gap in the evolution of cardiovascular diseases. Journal of Cellular Physiology, 236(9), 6282-6296. https://doi.org/10.1002/jcp.30345
Erten, M. (2021). Visfatin as a promising marker of cardiometabolic risk. Acta Cardiologica Sinica, 37(5), 464. https://doi.org/10.6515/ACS.202109_37(5).20210323B
Farghaly, H. S., Metwalley, K. A., Ahmed, F. A., Raafat, D. M., El-Asheer, O., Ali, A. M., ... & Zahran, A. M. (2017). Visfatin level in children and adolescents with autoimmune thyroiditis. Therapeutic advances in endocrinology and metabolism, 8(8), 119-125. https://doi.org/10.1177/2042018817731073
Fröhlich, E., & Wahl, R. (2017). Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Frontiers in immunology, 8, 521. https://doi.org/10.3389/fimmu.2017.00521
Ginabreda, M. G. P., Cardoso, L. C., Nobrega, F. M., Ferreira, A. C., Goncalves, M. D. C., Vaisman, M., & Carvalho, D. P. (2008). Negative correlation between thyroperoxidase and dual oxidase H2O2-generating activities in thyroid nodular lesions. European Journal of Endocrinology, 158(2), 223-227. https://doi.org/10.1530/EJE-07-0602
Hami, M. A., Jawzal, K. H., Mohammed, L. Y., & Ibrahiem, A. A. (2022). Studying the Association Between Systolic Blood Pressure and Thyroid Stimulating Hormone in Newly Diagnosed Subclinical Hyperthyroidism Female Patients. Science Journal of University of Zakho, 10(4), 197-200. https://doi.org/10.25271/sjuoz.2022.10.4.969
Irace, C., Carallo, C., Scavelli, F. B., De Franceschi, M. S., Esposito, T., Tripolino, C., & Gnasso, A. (2013). Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. International journal of clinical practice, 67(7), 665-672. https://doi.org/10.1111/ijcp.12124
Jawzal, K., Hami, M., Mohammed, L., & Ibrahiem, A. (2022). The relationship between thyroid hormones and lipid profile in subclinical hypothyroidism female patients. Baghdad Journal of Biochemistry and Applied Biological Sciences, 3(03), 200-209. https://doi.org/10.47419/bjbabs.v3i03.137
Kong, Q., Xia, M., Liang, R., Li, L., Cu, X., Sun, Z., & Hu, J. (2014). Increased serum visfatin as a risk factor for atherosclerosis in patients with ischaemic cerebrovascular disease. Singapore medical journal, 55(7), 383. https://doi.org/10.11622/smedj.2014091
Kweon, S. S., Shin, M. H., Nam, H. S., Jeong, S. K., Park, K. S., Choi, J. S., & Lee, Y. H. (2013). Sex Differences in the Associations of Testosterone and Sex Hormone-Binding Globulin With Metabolic Syndrome in Middle-Aged and Elderly Koreans–The Namwon Study–. Circulation Journal, 77(3), 734-740. https://doi.org/10.1253/circj.cj-12-0613
Luk, T., Malam, Z., & Marshall, J. C. (2008). Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. Journal of Leucocyte Biology, 83(4), 804-816. https://doi.org/10.1189/jlb.0807581
Leustean, L., Preda, C., Teodoriu, L., Mihalache, L., Arhire, L., & Ungureanu, M. C. (2021). Role of irisin in endocrine and metabolic disorders—possible new therapeutic agent?. Applied Sciences, 11(12), 5579. https://doi.org/10.3390/app11125579
Ma, X., Wang, F., Zhen, X., Zhao, L., Fang, L., Dong, Z., ... & Zhou, X. (2020). gp91phox, a novel biomarker evaluating oxidative stress, is elevated in subclinical hypothyroidism. International Journal of Endocrinology, 2020(1), 3161730. https://doi.org/10.1155/2020/3161730
Mahat, R. K., Panda, G., Nayak, B. P., & Panda, S. (2023). Association of vitamin D with triglyceride-glucose index and cardiometabolic risk factors in subclinical hypothyroidism. Human Nutrition & Metabolism, 34, 200226. https://doi.org/10.1016/j.hnm.2023.200226
Mancini, A., Di Segni, C., Raimondo, S., Olivieri, G., Silvestrini, A., Meucci, E., & Currò, D. (2016). Thyroid hormones, oxidative stress, and inflammation. Mediators of inflammation, 2016(1), 6757154. https://doi.org/10.1155/2016/6757154
Marotta, T., Russo, B. F., & Ferrara, L. A. (2010). Triglyceride‐to‐HDL‐cholesterol ratio and metabolic syndrome as contributors to cardiovascular risk in overweight patients. Obesity, 18(8), 1608-1613. https://doi.org/10.1038/oby.2009.446
Mayi, T. H., Duhem, C., Copin, C., Bouhlel, M. A., Rigamonti, E., Pattou, F., ... & Chinetti‐Gbaguidi, G. (2010). Visfatin is induced by peroxisome proliferator‐activated receptor gamma in human macrophages. The FEBS journal, 277(16), 3308-3320. https://doi.org/10.1111/j.1742-4658.2010.07729.x
Nilofer Sagana, M. K., Arul Senghor, K. A., Vinodhini, V. M., & P, R. (2024). Irisin and triglyceride glucose index as markers of dyslipidemia in young adults. Indian Journal of Clinical Biochemistry, 39(1), 136-141. https://doi.org/10.1007/s12291-022-01083-3
Onur Kirac, C., Sirikci, V., & Avni Findikli, H. (2022). Comparison of triglyceride-glucose index and HOMA-IR as indicators of insulin resistance in obese women with subclinical hypothyroidism. European Journal of Clinical and Experimental Medicine, (4), 412-416. http://dx.doi.org/10.15584/ejcem.2022.4.5
Öztürk, Ü., Vural, P., Özderya, A., Karadağ, B., Doğru-Abbasoğlu, S., & Uysal, M. (2012). Oxidative stress parameters in serum and low density lipoproteins of Hashimoto's thyroiditis patients with subclinical and overt hypothyroidism. International immunopharmacology, 14(4), 349-352. https://doi.org/10.1016/j.intimp.2012.08.010
Öztürk, Ü., Vural, P., Özderya, A., Karadağ, B., Doğru-Abbasoğlu, S., & Uysal, M. (2012). Oxidative stress parameters in serum and low density lipoproteins of Hashimoto's thyroiditis patients with subclinical and overt hypothyroidism. International immunopharmacology, 14(4), 349-352. https://doi.org/10.1016/j.intimp.2012.08.010
Razvi, S., Weaver, J. U., & Pearce, S. H. (2010). Subclinical thyroid disorders: significance and clinical impact. Journal of clinical pathology, 63(5), 379-386. https://doi.org/10.1136/jcp.2008.057414
Hasan, R. I., & Raziq, A. H. (2019). Studying the frequency of autoimmune thyroid diseases in Duhok Province. Science Journal of University of Zakho, 7(2), 45-49. https://doi.org/10.25271/sjuoz.2019.7.2.587
Sabharwal, R., Mahajan, P., & Bhatia, A. S. (2017). Association of subclinical hypothyroidism with dyslipidemia. JK Science, 19(2), 81-84. https://jkscience.org/archives/volume192/4-Original%20Article.pdf
Salih, S. F. (2022). Proprotein convertase subtilisin and kexin type 9 in Sub Clinical Hypothyroidism as a Risk Factor for Cardiovascular Disease. Journal of Pharmaceutical Negative Results, 13(3). https://doi.org/10.47750/pnr.2022.13.03.155
Sedeek, M., Hébert, R. L., Kennedy, C. R., Burns, K. D., & Touyz, R. M. (2009). Molecular mechanisms of hypertension: role of Nox family NADPH oxidases. Current opinion in nephrology and hypertension, 18(2), 122-127. https://doi.org/10.1097/mnh.0b013e32832923c3
Solanki, A., Bansal, S., Jindal, S., Saxena, V., & Shukla, U. S. (2013). Relationship of serum thyroid stimulating hormone with body mass index in healthy adults. Indian journal of endocrinology and metabolism, 17(Suppl1), S167-S169. https://doi.org/10.4103/2230-8210.119560
Suleiman, R. R., Salih, S. F., Abdullah, B. I., Ibrahim, I. H., & Saeed, Z. A. (2023). Triglyceride glucose index, its modified indices, and triglyceride HDL-C ratio as predictor markers of insulin resistance in prediabetic individuals. Medical Journal of Babylon, 20(2), 268-273. http://dx.doi.org/10.4103/MJBL.MJBL_269_22
Szanto, I., Pusztaszeri, M., & Mavromati, M. (2019). H2O2 metabolism in normal thyroid cells and in thyroid tumorigenesis: focus on NADPH oxidases. Antioxidants, 8(5), 126. https://doi.org/10.3390/antiox8050126
Ugur, K., Erman, F., Turkoglu, S., Aydin, Y., Aksoy, A., Lale, A., ... & Yalniz, M. (2022). Asprosin, visfatin and subfatin as new biomarkers of obesity and metabolic syndrome. European Review for Medical & Pharmacological Sciences, 26(6). https://doi.org/10.26355/eurrev_202203_28360
Walczak, K., & Sieminska, L. (2021). Obesity and thyroid axis. International journal of environmental research and public health, 18(18), 9434. https://doi.org/10.3390/ijerph18189434
Weir, C. B., & Jan, A. (2019). BMI classification percentile and cut off points. https://www.ncbi.nlm.nih.gov/books/NBK541070/
Wu, M. H., Tsai, C. H., Huang, Y. L., Fong, Y. C., & Tang, C. H. (2018). Visfatin promotes IL-6 and TNF-α production in human synovial fibroblasts by repressing miR-199a-5p through ERK, p38 and JNK signaling pathways. International journal of molecular sciences, 19(1), 190. https://doi.org/10.3390/ijms19010190
Yang, N., Zhang, H., Gao, X., Miao, L., Yao, Z., Xu, Y., & Wang, G. (2019). Role of irisin in Chinese patients with hypothyroidism: an interventional study. Journal of International Medical Research, 47(4), 1592-1601. https://doi.org/10.1177/0300060518824445
Zhang, Y., Murugesan, P., Huang, K., & Cai, H. (2020). NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nature Reviews Cardiology, 17(3), 170-194. https://doi.org/10.1038/s41569-019-0260-8
Zybek-Kocik, A., Sawicka-Gutaj, N., Wrotkowska, E., Sowiński, J., & Ruchała, M. (2016). Time-dependent irisin concentration changes in patients affected by overt hypothyroidism. Endokrynologia Polska, 67(5), 476-480. https://doi.org/10.5603/ep.a2016.0030
Zimmermann, M. B., & Boelaert, K. (2015). Iodine deficiency and thyroid disorders. The lancet Diabetes & endocrinology, 3(4), 286-295. https://doi.org/10.1016/s2213-8587(14)70225-6
Authors
Copyright (c) 2025 Barhav I. Abdullah , Sherwan F. Salih

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License [CC BY-NC-SA 4.0] that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work, with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online.