THE SYNTHESIS, CHARACTERIZATION, DFT-OPTIMIZATION, BIOLOGICAL ASSAYS, AND HEAVY METAL STUDIES OF A NEW TETRA DENTATE DERIVATIVE LIGAND AND ITS COMPLEXES
Abstract
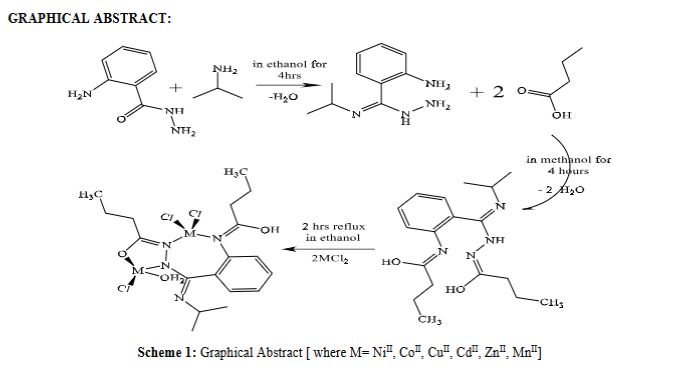
A new tetra dentate derivative ligand [(E)-N-((E)-(2-(((Z)-1-hydroxybutylidene) amino) phenyl) (isopropylimino) methyl) butyrohydrazonic acid] [N3O] type has been prepared from the condensation process of equimolar 2-aminobenzohydrazide, isopropyl amine and followed by another addition of butyric acid. Spectroscopic techniques as FT-IR, UV-visible, Mass spectrum, 1H,13C-NMR, T.L.C., Melting point, Conductivity measurements, Magnetic moment, DFT-optimization studies and other methods have been used to characterize the ligand and its new complexes with the general formula [L(M2)Cl3.H2O] (where M= NiII, CoII, CuII, MnII, CdII, and ZnII). Studying biological activity for the ligand and its complexes against two gram-positive and two gram-negative bacteria . The formed compounds were evaluated for antibacterial activity against two gram-positive and two gram-negative bacteria which are Staphylococcus aureus, Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa. The developed ligand and its metal complexes performed well versus both kinds of bacteria Scheme 1
Full text article
References
Abd El‐Halim, H. G. (2018). Antimicrobial and anticancer activities of Schiff base ligand and its transition metal mixed ligand complexes with heterocyclic base. Applied Organometallic Chemistry, 32(1), e3899. DOI: https://doi.org/10.1002/aoc.3899
Abu-Dief, A. M., & Mohamed, I. M. (2015). A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef University Journal of Basic and Applied Sciences, 4(2), 119-133. DOI:https://doi.org/10.1016/j.bjbas.2015.05.004
Akbari, Z. C.-D. (2024). Biological evaluation, DFT, MEP, HOMO-LUMO analysis and ensemble docking studies of Zn (II) complexes of bidentate and tetradentate Schiff base ligands as antileukemia agents. Journal of Molecular Structure. DOI:https://doi.org/10.1038/s41598-024-54021-z
Akkuş Taş, N. A. (2024). Synthesis, Enzyme Inhibition, and in Silico Studies of Amino Acid Schiff Bases. Iran. J. Chem. Chem. Eng.(IJCCE) Research Article Vol, 43(3). DOI: https://doi.org/10.1117/12.3034192
Ali, A., Pervaiz, M., Saeed, Z., Younas, U., Bashir, R., Ullah, S., . . . Rashid, A. (2022). Synthesis and biological evaluation of 4-dimethylaminobenzaldehyde derivatives of Schiff bases metal complexes: A review. Inorganic Chemistry Communications, 145, 109903. DOI:https://doi.org/10.1016/j.inoche.2022.109903
Alsalihi, E. I.-F. (2018). Synthesis and antibacterial activity of isatin Schiff base derivative with 3-aminoacetophenone and its Ni (II), Co (II) transition metals complexes. ARO-The Scientific Journal of Koya University, 6(1), 38-45. DOI:http://dx.doi.org/10.14500/aro.10245
Aran, M. M. (2025). Electrical conductance study of Schiff base in different solvents and temperatures: DFT calculation. Bulletin of the Chemical Society of Ethiopia, 39(1). DOI:10.4314/bcse.v39i1.15
Bain, G. A. (2008). Diamagnetic corrections and Pascal's constants. Journal of Chemical Education, 85(4), 532. DOI:https://doi.org/10.1021/ed085p532
Baroi, G. N., Gavala, H. N., Westermann, P., & Skiadas, I. (2017). Fermentative production of butyric acid from wheat straw: Economic evaluation. Industrial Crops and Products, 104, 68-80. DOI:https://doi.org/10.1016/j.indcrop.2017.04.008
Becke, A. D. (1993). Density‐functional thermochemistry. III. The role of exact exchange. The Journal of chemical physics, 98(7), 5648-5652. DOI:https://doi.org/10.1063/1.464913
Chérif, I. B. (2024). A theoretical and electrochemical impedance spectroscopy study of the adsorption and sensing of selected metal ions by 4-morpholino-7-nitrobenzofuran. Heliyon. DOI:10.1016/j.heliyon.2024.e26709
Diab, M. G. (2019). Sonbati, SM Morgan, S. Abbas, Inner metal complexes of tetradentate Schiff base: Synthesis, characterization, biological activity and molecular docking studies. Applied Organometallic Chemistry, 33(7). DOI:https://doi.org/10.1002/aoc.4945
Ghosh, P., Dey, S. K., Ara, M. H., Karim, K., & Islam, A. (2019). A review on synthesis and versatile applications of some selected Schiff bases with their transition metal complexes. Egyptian Journal of Chemistry, 62(Special Issue (Part 2) Innovation in Chemistry), 523-547. DOI: 10.21608/ejchem.2019.13741.1852
Hadi, M. K. (2022). Synthesis, characterization and preliminary antimicrobial evaluation of new schiff bases and aminothiadiazole derivatives of N-substituted phthalimide. Research Journal of Pharmacy and Technology, 15(9). DOI:10.52711/0974-360X.2022.00647
Hamad, A. A., Omer, R. A., Kaka, K. N., Abdulkareem, E. I., & Rashid, R. F. (2024). Biological activities of metal complexes with Schiff base. Reviews in Inorganic Chemistry(0). DOI:https://doi.org/10.1515/revic-2024-0075
Islam, M. R., & Mohsin, M. (2007). Synthesis of isatin, 5-chloroisatin and their∆-2-1, 3, 4 oxadiazoline derivatives for comparative cytotoxicity study on brine shrimp. ||| Bangladesh Journal of Pharmacology, 2(1), 7-12. DOI:https://doi.org/10.3329/bjp.v2i1.494
Kane, C. H., Tinguiano, D., Tamboura, F. B., Thiam, I. E., Barry, A. H., Gaye, M., & Retailleau, P. (2016). Synthesis and characterization of novel M (II)(M= Mn (II), Ni (II), Cu (II) or Zn (II)) complexes with tridentate N2, O-donor ligand (E)-2-amino-N’-[1-(pyridin-2-yl)-ethylidene] benzohydrazide. Bulletin of the Chemical Society of Ethiopia, 30(1), 101-110. DOI:10.4314/bcse.v30i1.9
Kumagai, S. I. (2024). Solid-state NMR of the retinal protonated Schiff base in microbial rhodopsins. Magnetic Resonance Letters, 4(3). DOI:https://doi.org/10.1016/j.mrl.2024.200132
Kumar, V. V., & Anthony, S. P. (2015). Heavy metal cation and anion sensing studies of N-(2-hydroxybenzyl)-isopropylamine surface functionalized AgNPs. New Journal of Chemistry, 39(2), 1308-1314. DOI:DOI https://doi.org/10.1039/C4NJ01740D
Lockyer, N. P.-T. (2024). Secondary ion mass spectrometry. Nature Reviews Methods Primers, 32. DOI:https://doi.org/10.1038/s43586-024-00311-9
Lundgren, R. J. (2016). Key concepts in ligand design: an introduction. Ligand Design in Metal Chemistry: Reactivity and Catalysis, 1-14. DOI:10.1002/9781118839621
Mukhtar, S. S., Hassan, A. S., Morsy, N. M., Hafez, T. S., Hassaneen, H. M., & Saleh, F. M. (2021). Overview on synthesis, reactions, applications, and biological activities of Schiff bases. Egyptian Journal of Chemistry, 64(11), 6541-6554. DOI: 10.21608/ejchem.2021.79736.3920
Nawaz, N., Ahmad, I., Darwesh, N. M., Wahab, A., Rahman, S. U., Khan, F. A., . . . Uddin, K. (2020). Synthesis, characterization and antioxidant activity of nickel (II) Schiff base complexes derived from 4-(dimethylamino) benzaldehyde. Journal of the Chemical Society of Pakistan, 42(2), 238-242. DOI:https://doi.org/10.1016/j.rechem.2024.101517
Parr, R. G. (1999). Electrophilicity index. Journal of the American Chemical Society, 121(9), 1922-1924. DOI:https://doi.org/10.1021/ja983494x
Ravichandran, R. M. (2014). Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food chemistry, 472-478. DOI:https://doi.org/10.1016/j.foodchem.2013.09.080
Reddy, K. H. (2007). Bioinorganic chemistry. New Age International. DOI:http://13.232.72.61:8080/jspui/handle/123456789/559
Senthilkumar, S. J. (2021). Synthesis, structure analysis, biological activity and molecular docking studies of some hydrazones derived from 4-aminobenzohydrazide. Journal of Molecular Structure, 1226, 129354. DOI:https://doi.org/10.1016/j.molstruc.2020.129354
Singh, G. J. (2021). Synthesis, characterization and UV–visible study of schiff base-acetylene functionalized organosilatrane receptor for the dual detection of Zn2+ and Co2+ ions. Inorganica Chimica Acta, 525. DOI:https://doi.org/10.1016/j.ica.2021.120465
Tanabe, Y. S. (1954). On the absorption spectra of complex ions II. Journal of the Physical Society of Japan, 9(5). DOI:https://doi.org/10.1143/JPSJ.9.766
Wang, H.-C., Yan, X.-Q., Yan, T.-L., Li, H.-X., Wang, Z.-C., & Zhu, H.-L. (2016). Design, synthesis and biological evaluation of benzohydrazide derivatives containing dihydropyrazoles as potential EGFR kinase inhibitors. Molecules, 21(8), 1012. DOI:https://doi.org/10.3390/molecules21081012
Xiao, Z. C.-T. (2018). Production of butyric acid from acid hydrolysate of corn husk in fermentation by Clostridium tyrobutyricum: kinetics and process economic analysis. Biotechnology for biofuels, 11. DOI:https://doi.org/10.1186/s13068-018-1165-1
Zaman Brohi, R. O. (2020). Graphene oxide functionalized with a Schiff Base for the removal of Pb (II) ions from contaminated water: experimental and modeling approach. Journal of Chemical Technology & Biotechnology, 95(6). DOI:https://doi.org/10.1002/jctb.6362
Authors
Copyright (c) 2025 Kwestan Namiq Aziz , Eman Ibrahim Alsalihi

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License [CC BY-NC-SA 4.0] that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work, with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online.