EXPRESSION LEVELS OF ENDOSOMAL TOLL-LIKE RECEPTORS (TLRs) IN RHEUMATOID ARTHRITIS: A LINK BETWEEN TLR7, TLR9, AND INFLAMMATORY BIOMARKERS WITH DISEASE ACTIVITY SCORE
Abstract
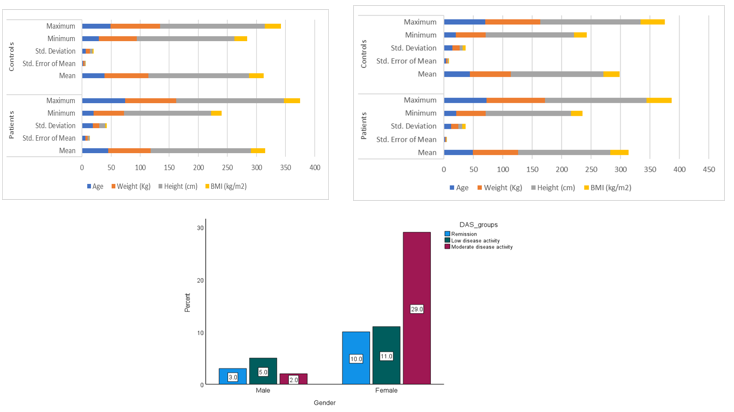
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by synovial inflammation, joint damage, and functional impairment. Recent studies highlighted the role of Toll-like receptors (TLRs), particularly TLR7 and TLR9, in RA pathogenesis through innate immune activation and interferon-alpha (IFN-α) production. This case-control study assessed TLR7 and TLR9 expression in 60 RA patients and 30 healthy controls, alongside serum IFN-α levels and disease activity (DAS28). Blood samples were processed for RNA extraction and cDNA synthesis. Gene expression was quantified via RT-qPCR, and IFN-α levels measured by ELISA. RA patients showed significantly increased TLR7 (1.99-fold, p = 0.005) and TLR9 (1.42-fold, p = 0.024) expression compared to healthy controls, as determined by the Mann–Whitney U test. A strong positive correlation was observed between their expression levels (ρ = 0.893, p ≤ 0.001), indicating shared co-regulatory mechanisms. IFN-α levels were also significantly elevated in RA patients (p ≤ 0.001). However, correlations between TLR7 expression and IFN-α (ρ = –0.047, p = 0.804) or DAS28 (ρ = 0.148, p = 0.435) were weak and not statistically significant. Similarly, TLR9 expression showed weak, non-significant correlations with IFN-α (ρ = 0.007, p = 0.969) and DAS28 (ρ = 0.161, p = 0.396). Likewise, no significant link was found between age and disease activity. These results suggest that while TLR7, TLR9, and IFN-α are elevated in RA, their direct association with disease activity may be limited, highlighting the complexity of immune regulatory networks in RA progression.
Full text article
References
Ashai, S., & Harvey, N. C. (2020). Rheumatoid Arthritis and Bone Health. Clinical Medicine 20(6), 565–567. https://doi.org/10.7861/clinmed.20.6.rabh
Asuzu-Samuel, H. O. (2021). The prevalence of rheumatoid arthritis on female patients of child-bearing age at University of Port Harcourt Teaching Hospital, Rivers State, Nigeria. GSC Advanced Research and Reviews, 8(2), 1–7. https://doi.org/10.30574/gscarr.2021.8.2.0163
Baker, R., Mantilla, B., Graf, J., Katz, P. P., Goglin, S., Barton, J. L., Liew, J. W., & Wysham, K. D. (2023). Racial and ethnic differences in a biochemical marker of rheumatoid arthritis disease activity. ACR Open Rheumatology, 5(3), 142–148. https://doi.org/10.1002/acr2.11524
Duffy, L., & O’Reilly, S. C. (2016). Toll-like receptors in the pathogenesis of autoimmune diseases: Recent and emerging translational developments. ImmunoTargets and Therapy, 5, 69–80.https://doi.org/10.2147/ITT.S89795
Edilova, M. I., Akram, A., & Abdul-Sater, A. A. (2021). Innate immunity drives pathogenesis of rheumatoid arthritis. Biomedical Journal, 44(2), 172–182https://doi.org/10.1016/j.bj.2020.06.010
Fattahi, M. J., & Mirshafiey, A. (2012). Prostaglandins and rheumatoid arthritis. Arthritis, 2012, 239310. https://doi.org/10.1155/2012/239310
Firestein, G. S., & McInnes, I. B. (2017). Immunopathogenesis of rheumatoid arthritis. Immunity, 46(2), 183–196. https://doi.org/10.1016/j.immuni.2017.02.006
Greenmyer, J. R., Stacy, J. M., Sahmoun, A. E., Beal, J. R., & Diri, E. (2020). DAS28‐CRP cutoffs for high disease activity and remission are lower than DAS28‐ESR in rheumatoid arthritis. ACR Open Rheumatology, 2(9), 507–511.https://doi.org/10.1002/acr2.11171
Hansen, I. M. J., Andreasen, R. A., Hansen, M. N. B., & Emamifar, A. (2017). The reliability of disease activity score in 28 joints–C-reactive protein might be overestimated in a subgroup of rheumatoid arthritis patients, when the score is solely based on subjective parameters: A cross-sectional, exploratory study. Journal of Clinical Rheumatology, 23(2), 102–106. https://doi.org/10.1097/RHU.0000000000000469
Hurst, J., & von Landenberg, P. (2008). Toll-like receptors and autoimmunity. Autoimmunity Reviews, 7(3), 204–208. https://doi.org/10.1016/j.autrev.2007.11.006
Jahid, M., Khan, K. U., & Ahmed, R. S. (2023). Overview of rheumatoid arthritis and scientific understanding of the disease. Mediterranean Journal of Rheumatology, 34(3), 284–291. https://doi.org/10.31138/mjr.20230801.oo
Kalliolias, G. D., Basdra, E. K., & Papavassiliou, A. G. (2024). Targeting TLR signaling cascades in systemic lupus erythematosus and rheumatoid arthritis: An update. Biomedicines, 12(1), 138. https://doi.org/10.3390/biomedicines12010138
Kawasaki, T., & Kawai, T. (2014). Toll-like receptor signaling pathways. Frontiers in Immunology, 5, 461. https://doi.org/10.3389/fimmu.2014.00461
Kvien, T. K., Uhlig, T., Ødegård, S., & Heiberg, M. S. (2006). Epidemiological aspects of rheumatoid arthritis: The sex ratio. Annals of the New York Academy of Sciences, 1069(1), 212–222. https://doi.org/10.1196/annals.1351.019
Letarouilly, J. G., Flipo, R. M., Cortet, B., Tournadre, A., & Paccou, J. (2021). Body composition in patients with rheumatoid arthritis: A narrative literature review. Therapeutic Advances in Musculoskeletal Disease, 13, 1759720X211015006. https://doi.org/10.1177/1759720x211015006
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods, 25(4), 402–408. https://doi.org/10.1006/meth.2001.1262
Mathkhor, A. J., Mardan, F. T., & Allawi, A. B. (2022). Prevalence and impact of obesity in patients with rheumatoid arthritis. Rheumatology International. https://doi.org/10.46439/rheumatology.3.020
Matsui, T., Kuga, Y., Kaneko, A., Nishino, J., Eto, Y., Chiba, N., Yasuda, M., et al. (2007). Disease activity score 28 (DAS28) using C-reactive protein underestimates disease activity and overestimates EULAR response criteria compared with DAS28 using erythrocyte sedimentation rate in a large observational cohort of rheumatoid arthritis patients in Japan. Annals of the Rheumatic Diseases, 66(9), 1221–1226. https://doi.org/10.1136/ard.2006.063834
Michielsens, C. A.J., T. E. Bolhuis, F. A. van Gaalen, F. H.J. van den Hoogen, L. M. Verhoef, N. den Broeder, and A. A. den Broeder. 2024. ‘Construct Validity of Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Ankylosing Spondylitis Disease Activity Score (ASDAS) Treatment Target Cut-Offs in a BASDAI Treat-to-Target Axial Spondyloarthritis Cohort: A Cross-Sectional Study’. Scandinavian Journal of Rheumatology 53(3): 180–87. https://doi.org/10.1080/03009742.2023.2213509
Okada, Y., Eyre, S., Suzuki, A., Kochi, Y., & Yamamoto, K. (2019). Genetics of rheumatoid arthritis: 2018 status. Annals of the Rheumatic Diseases, 78(4), 446–453. https://doi.org/10.1136/annrheumdis-2018-213678
Piccinni, M. P., Lombardelli, L., Logiodice, F., Kullolli, O., Parronchi, P., & Romagnani, S. (2016). How pregnancy can affect autoimmune diseases progression? Clinical and Molecular Allergy, 14, 1–9. https://doi.org/10.1186/s12948-016-0048-x
Radakovics, K., Battin, C., Leitner, J., Geiselhart, S., Paster, W., Stöckl, J., Hoffmann-Sommergruber, K., & Steinberger, P. (2022). A highly sensitive cell-based TLR reporter platform for the specific detection of bacterial TLR ligands. Frontiers in Immunology, 12, 817604. https://doi.org/10.3389/fimmu.2021.817604
Ramos-González, E. J., Bastian, Y., Castañeda-Delgado, J. E., Zapata-Zúñiga, M., Gómez-Moreno, M., Castillo-Ortiz, J. D., Ramos-Remus, C., & Enciso-Moreno, J. A. (2022). Overexpression of TLR7 and TLR9 occurs before onset symptoms in first-degree relatives of rheumatoid arthritis patients. Archives of Medical Research, 53(1), 86–92. https://doi.org/10.1016/j.arcmed.2021.06.010
Rastogi, A., Algulin, J., Mangat, P., Lim, A. K. P., Satchithananda, K., Hajnal, J. V., & Taylor, P. C. (2015). Early metacarpal bone mineral density loss using digital X‐ray radiogrammetry and 3‐Tesla wrist MRI in established rheumatoid arthritis: A longitudinal one‐year observational study. Arthritis, 2015, 852989. https://doi.org/10.1155/2015/852989
Smolen, J., Pitzalis, C., Jones, S., & McKenna, F. (2016). Think rheumatoid arthritis: Causes, consequences, and management. EMJ Rheumatology, 3(1), 66–73. https://doi.org/10.33590/emjrheumatol/10314552
Son, K. M., Kim, S. Y., Lee, S. H., Yang, C. M., Seo, Y. I., & Kim, H. A. (2016). Comparison of the disease activity score using the erythrocyte sedimentation rate and C‐reactive protein levels in Koreans with rheumatoid arthritis. International Journal of Rheumatic Diseases, 19(12), 1278–1283. https://doi.org/10.1111/1756-185X.12843
Soták, M., Clark, M., Suur, B. E., & Börgeson, E. (2025). Inflammation and resolution in obesity. Nature Reviews Endocrinology, 21(1), 45–61. https://doi.org/10.1038/s41574-024-01047-y
Suta, C., Petcu, L., Craiu, E., & Suta, M. (2015). Sex ratio and age in patients with rheumatoid arthritis: Data from a cohort in South-East Romania. Romanian Journal of Rheumatology/Revista Romana de Reumatologie, 24(4). https://rjr.com.ro/rjr-vol-xxiv-no-4-year-2015/
Swain, N., Tripathy, A., Padhan, P., Raghav, S. K., & Gupta, B. (2022). Toll-like receptor-7 activation in CD8+ T cells modulates inflammatory mediators in patients with rheumatoid arthritis. Rheumatology International, 42(7), 1235–1245. https://doi.org/10.1007/s00296-021-05050-8
Van Riel, P. L., & Renskers, L. (2016). The disease activity score (DAS) and the disease activity score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clinical and Experimental Rheumatology, 34(5 Suppl 101), S40–S44. https://www.clinexprheumatol.org/abstract.asp?a=11135
van Vollenhoven, R. F. (2009). Sex differences in rheumatoid arthritis: More than meets the eye. BMC Medicine, 7, 12. https://doi.org/10.1186/1741-7015-7-12
Venetsanopoulou, A. I., Alamanos, Y., Voulgari, P. V., & Drosos, A. A. (2023). Epidemiology and risk factors for rheumatoid arthritis development. Mediterranean Journal of Rheumatology, 34(4), 404. https://mjrheum.org/assets/files/792/file525_1799.pdf
Wells, G., Becker, J. C., Teng, J., Dougados, M., Schiff, M., Smolen, J., Aletaha, D., & Van Riel, P. L. (2009). Validation of the 28-joint disease activity score (DAS28) and European League Against Rheumatism response criteria based on C-reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Annals of the Rheumatic Diseases, 68(6), 954–960. https://doi.org/10.1136/ard.2007.084459
Weyand, C. M., & Goronzy, J. J. (2020). Immunometabolism in the development of rheumatoid arthritis. Immunological Reviews, 294(1), 177–187. https://doi.org/10.1111/imr.12838
Zefenkey, Z. (2022). Influence of Helicobacter pylori infection on atherosclerosis risk factors in older people. Science Journal of University of Zakho, 10(1), 24–28. https://doi.org/10.25271/sjuoz.2022.10.1.884
Authors
Copyright (c) 2026 Dilzheen Muslih Salih and Amir Hani Raziq

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License [CC BY-NC-SA 4.0] that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work, with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online.