Effect of reduced folate carrier gene polymorphism (G80A) on the methotrexate level in patients with rheumatoid arthritis and it’s relation to the disease activity
Abstract
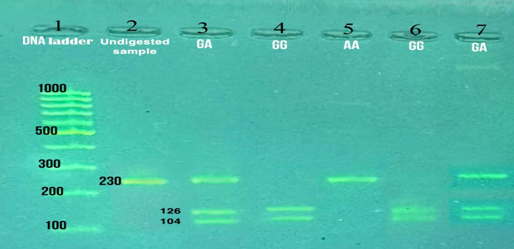
Methotrexate (MTX) is the primary conventional synthetic disease-modifying anti-rheumatic drug (csDMARD) used to treat rheumatoid arthritis (RA). Interindividual variability in its efficacy and toxicity has been partially attributed to genetic polymorphisms affecting folate metabolism and transport. The reduced folate carrier-1 (RFC1), encoded by the SLC19A1 gene, is a key transporter responsible for MTX cellular uptake. The RFC1 G80A (rs1051266) polymorphism may alter transporter function, influencing MTX plasma levels and therapeutic outcomes. This study attempted to evaluate the impact of RFC1 G80A polymorphism on MTX plasma concentration and its association with the activity of RA disease. This cross-sectional study was carried out at the Rheumatology Center in Duhok, Iraq, from September 2024 to June 2025. Ninety RA patients receiving oral MTX therapy were recruited. MTX levels were quantified by ELISA, and genotyping of the RFC1 G80A polymorphism was performed by PCR-RFLP. Statistical analyses were conducted to evaluate genotype associations with MTX levels, DAS-28 activity score, and laboratory parameters. The study cohort (mean age: 50.1 ± 11.9 years) was predominantly female (91.1%). Genotype frequencies were GG (53.3%), GA (35.6%), and AA (11.1%). Although MTX levels were numerically highest in AA individuals (330.1 ± 70.5 ng/mL), the difference was not significant in statistical terms (p=0.82). However, AA carriers had significantly higher DAS-28 scores (4.3 ± 1.5) than GG carriers (3.8 ± 1.2; p=0.009), suggesting poorer disease control. No substantial variation has been noticed in inflammatory or hematological parameters between genotype groups. The RFC1 G80A polymorphism may influence MTX pharmacokinetics and RA disease activity. The AA genotype appears to be associated with non-significantly higher MTX levels and worse disease control. Pharmacogenetic profiling could help optimize MTX therapy in RA.
Full text article
References
References:
Alamanos, Y., & Drosos, A. A. (2005). Epidemiology of adult rheumatoid arthritis. Autoimmunity
reviews, 4(3), 130-136- doi: 10.1016/j.autrev.2004.09.002.
Aletaha, D., Neogi, T., Silman, A. J., Funovits, J., Felson, D. T., Bingham III, C. O., ... & Hawker, G. (2010). 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis & rheumatism, 62(9), 2569-2581. doi. 10.1002/art.27584
Brown, P. M., Pratt, A. G., & Isaacs, J. D. (2016). Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nature Reviews Rheumatology, 12(12), 731-742- doi: 10.1038/nrrheum.2016.175
Bullock, J., Rizvi, S. A., Saleh, A. M., Ahmed, S. S., Do, D. P., Ansari, R. A., & Ahmed, J. (2019). Rheumatoid arthritis: a brief overview of the treatment. Medical Principles and Practice, 27(6), 501-507- doi: 10.1159/000493390
Chango, Abalo, Nathalie Emery-Fillon, Géneviève Potier de Courcy, Daniel Lambert, Michèle Pfister, David S. Rosenblatt, and Jean-Pierre Nicolas. "A polymorphism (80G-> A) in the reduced folate carrier gene and its associations with folate status and homocysteinemia." Molecular genetics and metabolism 70, no. 4 (2000): 310-315- doi: 10.1006/mgme.2000.3034
Chiusolo, P., Giammarco, S., Bellesi, S., Metafuni, E., Piccirillo, N., De Ritis, D., ... & Sica, S. (2012). The role of MTHFR and RFC1 polymorphisms on toxicity and outcome of adult patients with hematological malignancies treated with high-dose methotrexate followed by leucovorin rescue. Cancer chemotherapy and pharmacology, 69, 691-696- doi: 10.1007/s00280-011-1751- 4
Das, S., & Padhan, P. (2017). An overview of the extraarticular involvement in rheumatoid arthritis and its management. Journal of Pharmacology and Pharmacotherapeutics, 8(3), 81-86- doi: 10.4103/jpp.JPP_194_16
Dervieux, T., Furst, D., Lein, D. O., Capps, R., Smith, K., Walsh, M., & Kremer, J. (2004). Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology, 50(9), 2766-2774- doi:10.1002/art.20460
Fleige, S., & Pfaffl, M. W. (2006). RNA integrity and the effect on the real-time qRT-PCR performance. Molecular aspects of medicine, 27(2-3), 126-139. doi: 10.1016/j.mam.2005.12.003
Kung, T. N., Dennis, J., Ma, Y., Xie, G., Bykerk, V., Pope, J., ... & Gagnon, F. (2014). RFC1 80G> A is a genetic determinant of methotrexate efficacy in rheumatoid arthritis: a human genome epidemiologic review and meta‐analysis of observational studies. Arthritis & Rheumatology, 66(5), 1111-1120- doi: 10.1002/art.38331.
Lv, S., Fan, H., Yang, H., Huang, J., Li, J., Shu, X., ... & Xiao, C. (2019). Membrane‐Spanning Protein Genetic Polymorphisms Related to Methotrexate Therapeutic Outcomes in a Chinese Rheumatoid Arthritis Population. The Journal of Clinical Pharmacology, 59(11), 1471-1476. doi: 10.1002/jcph.1446
Miller, S. A., Dykes, D. D., & Polesky, H. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic acids research, 16(3), 1215. doi: 10.1093/nar/16.3.1215
Nomair, A. M., Abdelati, A., Dwedar, F. I., Elnemr, R., Kamel, Y. N., & Nomeir, H. M. (2024). The impact of folate pathway variants on the outcome of methotrexate therapy in rheumatoid arthritis patients. Clinical Rheumatology, 43(3), 971-983- doi: 10.1007/s10067-024-06892-w
Owen, S. A., Hider, S. L., Martin, P., Bruce, I. N., Barton, A., & Thomson, W. (2013). Genetic polymorphisms in key methotrexate pathway genes are associated with response to treatment in rheumatoid arthritis patients. The pharmacogenomics journal, 13(3), 227-234- doi: 10.1038/tpj.2012.7
Prevoo, M. L. L., Van'T Hof, M., Kuper, H. H., Van Leeuwen, M. A., Van De Putte, L. B. A., & Van Riel, P. L. C. M. (1995). Modified disease activity scores that include twenty‐eight‐joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology, 38(1), 44-48. doi: 10.1002/art.1780380107
Ranganathan, P., & McLeod, H. L. (2006). Methotrexate pharmacogenetics: the first step toward individualized therapy in rheumatoid arthritis. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology, 54(5), 1366-1377- doi: 10.1002/art.21762.
Stamp, L. K., Barclay, M. L., O’DONNELL, J. L., Zhang, M., Drake, J., Frampton, C., & Chapman, P. T. (2011). Effects of changing from oral to subcutaneous methotrexate on red blood cell methotrexate polyglutamate concentrations and disease activity in patients with rheumatoid arthritis. The Journal of rheumatology, 38(12), 2540-2547.doi: 10.3899/jrheum.110481.
Stamp, L., Roberts, R., Kennedy, M., Barclay, M., O’Donnell, J., & Chapman, P. (2006). The use of low dose methotrexate in rheumatoid arthritis—are we entering a new era of therapeutic drug monitoring and pharmacogenomics?. Biomedicine & pharmacotherapy, 60(10), 678-687.doi: 10.1016/j.biopha.2006.09.007.
Szostak, B., Machaj, F., Rosik, J., & Pawlik, A. (2020). Using pharmacogenetics to predict methotrexate response in rheumatoid arthritis patients. Expert Opinion on Drug Metabolism & Toxicology, 16(7), 617–626- doi: 10.1080/17425255.2020.1777279
Wessels, J. A., de Vries‐Bouwstra, J. K., Heijmans, B. T., Slagboom, P. E., Goekoop‐Ruiterman, Y. P., Allaart, C. F., ... & Guchelaar, H. J. (2006). Efficacy and toxicity of methotrexate in early rheumatoid arthritis are associated with single‐nucleotide polymorphisms in genes coding for folate pathway enzymes. Arthritis & Rheumatism, 54(4), 1087-1095. doi: 10.1002/art.21726.
Wessels, J. A., van der Kooij, S. M., le Cessie, S., Kievit, W., Barerra, P., Allaart, C. F., ... & Pharmacogenetics Collaborative Research Group. (2007). A clinical pharmacogenetic model to predict the efficacy of methotrexate monotherapy in recent‐onset rheumatoid arthritis. Arthritis & Rheumatism, 56(6), 1765-1775. doi: 10.1002/art.22640.
Authors
Copyright (c) 2026 Rozheen Ghiath Jaafar and Adil Abozaid Eissa

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License [CC BY-NC-SA 4.0] that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work, with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online.