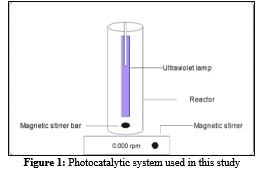
PHOTOCATALYTIC DEGRADATION OF FAST GREEN DYE IN AQUEOUS SOLUTION USING TITANIUM DIOXIDE AS PHOTOCATALYST
Abstract
Research on the photocatalytic degradation of dyes and other organic pollutants become an interesting topic in the last two decades. Under UV irradiation, investigation and optimization of various experimental parameters were done for the photocatalytic degradation of fast green dye (FG) in the presence of titanium dioxide (TiO2) suspension. Initial dye concentration, photocatalyst dosage, and solution pH were the study parameters. Results showed that the rate at which FG dye degraded was accelerated by increasing the TiO2 dose. However, the degradation rate was adversely impacted by the rise in FG dye content from 3 to 9 mg/L. It was found that the highest percentage of color removal occurred when the pH of the medium was 6.5. FG was found to be degraded process reached equilibrium in 50 minutes using TiO2 photocatalyst, proving that it is an effective catalyst for the process. Additionally, the kinetics of FG dye degradation was studied, and it was followed by the pseudo first-order kinetics.
Full text article
References
REFERENCE
Aguedach, A., Brosillon, S., & Morvan, J. (2005). Photocatalytic degradation of azo-dyes reactive black 5 and reactive yellow 145 in water over a newly deposited titanium dioxide. Applied Catalysis B: Environmental, 57(1), 55-62. https://doi.org/https://doi.org/10.1016/j.apcatb.2004.10.009
Benjelloun, M., Miyah, Y., Evrendilek, G. A., Zerrouq, F., & Lairini, S. (2021). Recent advances in adsorption kinetic models: their application to dye types. Arabian Journal of Chemistry, 14(4), 103031. https://doi.org/https://doi.org/10.1016/j.arabjc.2021.103031
Haji, A. A., & Mohammed, N. M. S. (2019). Optimization of arsenic adsorption onto activated carbon of potato peel using response surface methodology. Science Journal of University of Zakho, 7(2), 37-44. https://doi.org/https://doi.org/10.25271/sjuoz.2019.7.2.594
Idrees, S. A., Jamil, L. A., & Omer, K. M. (2023). Efficient photo-Fenton catalysis using magnetic iron nanoparticles decorated boron nitride quantum dots: theoretical and experimental investigations. RSC advances, 13(10), 6779-6792. https://doi.org/https://doi.org/10.1039/D3RA00234A
Idrees, S. A., Naman, S., & Shorachi, A. (2018). Kinetic and thermodynamic study of Trifluralin photo-degradation by ultra violet light. IOP Conference Series: Materials Science and Engineering,
Idrees, S. A., Salih, R. N., Bashir, K., & Hamasaeed, A. A. (2021). Kinetic Study of Congo-Red Photo-Catalytic Degradation in Aqueous Media Using Zinc Oxide as Photo Catalyst Under Led Light. Science Journal of University of Zakho, 9(1), 20-24. https://doi.org/https://doi.org/10.25271/sjuoz.2021.9.1.777
Kumar, A., Paliwal, M., Ameta, R., & Ameta, S. (2008). Oxidation of fast green FCF by the solar photo-Fenton process. Journal of the Iranian Chemical Society, 5, 346-351. https://doi.org/doi.org/10.1007/BF03246129
Kumar, M., Kumar, V., & Singh, R. (2017). Diameter tuning of β-Ga2O3 nanowires using chemical vapor deposition technique. Nanoscale Research Letters, 12(1), 184. https://doi.org/doi.org/10.1186/s11671-017-1915-1
Molla-Babaker, M. M., & Idreesb, S. A. (2020). Degradation of Congo Red Dye Using Homogeneous Photo Fenton Catalyst Coupled with Oxygen Kinetics and Statistical Analysis. Asian J. Appl. Chem. Res, 6(1), 1-9. https://doi.org/10.9734/AJACR/2020/v6i130147
Petkowicz, D. I., Pergher, S. B., Da Silva, C. D. S., Da Rocha, Z. N., & Dos Santos, J. H. (2010). Catalytic photodegradation of dyes by in situ zeolite-supported titania. Chemical Engineering Journal, 158(3), 505-512. https://doi.org/https://doi.org/10.1016/j.cej.2010.01.039
Rauf, M., Meetani, M., & Hisaindee, S. (2011). An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination, 276(1-3), 13-27. https://doi.org/https://doi.org/10.1016/j.desal.2011.03.071
SALIH, R. N., HAJI, A. A., KHALO, I. S., & AHMED, H. A. (2023). PHOTOCATALYTIC DEGRADATION OF EOSIN Yellowish DYE IN AQUEOUS SOLUTION USING TITANIUM DIOXIDE AS CATALYST: IMPACT OF VARIOUS PARAMETERS AND KINETIC STUDY. Journal of Duhok University, 26(2), 24-35. https://doi.org/https://doi.org/10.26682/sjuod.2023.26.2.3
Salih, R. N., & Naman, S.-A. (2019). photodegradation of 2, 4, 5, 6-tetrachloroisophthalonitrile (chlorothalonil) by visible and ultraviolet light in the presence of Zno and TiO2. Science Journal of University of Zakho, 7(3), 79-88. https://doi.org/https://doi.org/10.25271/sjuoz.2019.7.3.605
Yang, L., Liya, E. Y., & Ray, M. B. (2008). Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water research, 42(13), 3480-3488. https://doi.org/https://doi.org/10.1016/j.watres.2008.04.023
Zollinger, H. (2003). Color chemistry: syntheses, properties, and applications of organic dyes and pigments. John Wiley & Sons.
Authors
Copyright (c) 2025 Akram Haji, Akram A Haji , and Idrees S khalo

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License [CC BY-NC-SA 4.0] that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work, with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online.