USING INFRARED THERMOGRAPHIC TECHNIQUE AS AN ALTERNATIVE TO CONVENTIONAL RECTAL THERMOMETER IN SHEEP
Abstract
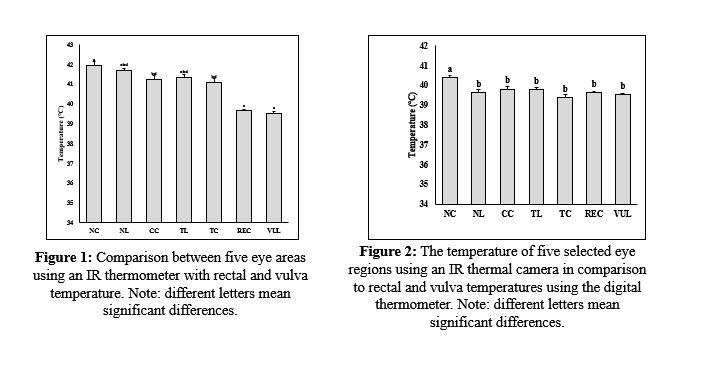
This research was established to assess the precision of the infrared (IR) camera and thermometer in measuring different body regions of sheep. This study was undertaken in the animal farm at the College of Agricultural Engineering Sciences, University of Duhok, Kurdistan Region of Iraq. The temperature was measured from the rectum using a digital thermometer. At the same time, the peripheral temperatures were measured from the ears, muzzle (MUZ), inner thigh (IT), inguinal region (ING), rectum, vulva, and five areas of the eyes, including nasal conjunctiva (NC), nasal limbus (NL), center cornea (CC), temporal limbus (TL), and temporal conjunctiva (TC) using IR thermometer and IR camera. The temperatures obtained from the IR thermometer were higher than those obtained from the digital thermometer. only NC temperature was found to be significantly (P<0.05) higher than other eye regions, and rectal and vulva temperatures. IR thermal camera showed no significant differences in the eyes' NL, CC, TL, and TC regions compared to rectum and vulva temperatures using the digital thermometer. The muzzle temperature was significantly (P<0.05) lower than all other body parts. In addition, the inner thigh, inguinal region, and ear temperatures were significantly (P<0.05) higher than rectum and vulva temperatures. The rectum and vulva temperatures obtained from the IR thermometer were significantly higher (P<0.05) than thermal camera and digital thermometer temperatures. In conclusion, thermal cameras as a non-invasive and accurate method can be an alternative temperature measuring method.
Full text article
References
Arfuso, F., Acri, G., Piccione, G., Sansotta, C., Fazio, F., Giudice, E., & Giannetto, C. (2022). Eye surface infrared thermography usefulness as a noninvasive method of measuring stress response in sheep during shearing: Correlations with serum cortisol and rectal temperature values. Physiology & Behavior, 250, 113781. https://doi.org/10.1016/j.physbeh.2022.113781
Cai, Z., Cui, J., Yuan, H., & Cheng, M. (2023). Application and research progress of infrared thermography in temperature measurement of livestock and poultry animals: A review. Computers and Electronics in Agriculture, 205, 107586. https://doi.org/10.1016/j.compag.2022.107586
Crawford, D. C., Hicks, B., & Thompson, M. J. (2006). Which thermometer? Factors influencing best choice for intermittent clinical temperature assessment. Journal of medical engineering & technology, 30(4), 199-211. https://doi.org/10.1080/03091900600711464
Fuchs, B., Sørheim, K. M., Chincarini, M., Brunberg, E., Stubsjøen, S. M., Bratbergsengen, K., Hvasshovd, S.O., Zimmermann, B., Lande, U.S. & Grøva, L. (2019). Heart rate sensor validation and seasonal and diurnal variation of body temperature and heart rate in domestic sheep. Veterinary and animal science, 8, 100075. https://doi.org/10.1016/j.vas.2019.100075
George, W. D., Godfrey, R. W., Ketring, R. C., Vinson, M. C., & Willard, S. T. (2014). Relationship among eye and muzzle temperatures measured using digital infrared thermal imaging and vaginal and rectal temperatures in hair sheep and cattle. Journal of Animal Science, 92(11), 4949-4955. https://doi.org/10.2527/jas.2014-8087
Goodwin, S. D. (1998). Comparison of body temperatures of goats, horses, and sheep measured with a tympanic infrared thermometer, an implantable microchip transponder, and a rectal thermometer. Journal of the American Association for Laboratory Animal Science, 37(3), 51-55.
Hussein, N. J., & Al-Nakshabendy, A. A. (2023). Can facial expressions and infrared thermography be used to measure positive emotions in goats?. Bulg J Agric Sci, 29, 733-739. https://www.agrojournal.org/29/04-21.html
Hussein, N. J., & Al-Naqshabendy, A. A. (2024a). USING SHEEP FACIAL GRIMACE SCALE, INFRARED THERMOGRAPHY AND CORTISOL HORMONE TO MEASURE PAIN IN SHEEP INFECTED WITH MASTITIS DISEASE. Science Journal of University of Zakho, 12(1), 70-74. https://doi.org/10.25271/sjuoz.2024.12.1.1185
HUSSEIN, N., & AL-NAQSHABENDY, A. A. (2024b). MEASURING POSITIVE EMOTIONS IN FREE-RANGE SHEEP USING PERIPHERAL TEMPERATURES, FACIAL ACTION UNITS AND EAR POSTURES. Bulgarian Journal of Veterinary Medicine, 27(4). https://doi.org/10.15547/bjvm.2022-0123
Ibáñez, C., Moreno-Manrique, M., Villagrá, A., Bueso-Ródenas, J., & Mínguez, C. (2023). Evaluation of Non-Contact Device to Measure Body Temperature in Sheep. Animals, 14(1), 98. https://doi.org/10.3390/ani14010098
Katsoulos, P. D., Athanasiou, L. V., Karatzia, M. A., Valasi, I., Boscos, C., & Karatzias, H. (2016). Comparison of a non-contact infrared thermometer with a rectal digital thermometer for use in ewes. Small Ruminant Research, 143, 84-88. https://doi.org/10.1016/j.smallrumres.2016.09.004
Knízková, İ., Kunc, P., Gürdil, G., Pınar, Y., & Selvi, K. Ç. (2007). Applications of infrared thermography in animal production. Anadolu Tarım Bilimleri Dergisi, 22(3), 329-336. https://doi.org/10.7161/anajas.2007.22.3.329-336
McLennan, K. M., Rebelo, C. J., Corke, M. J., Holmes, M. A., Leach, M. C., & Constantino-Casas, F. (2016). Development of a facial expression scale using footrot and mastitis as models of pain in sheep. Applied Animal Behaviour Science, 176, 19-26. https://doi.org/10.1016/j.applanim.2016.01.007
McManus, C., Tanure, C. B., Peripolli, V., Seixas, L., Fischer, V., Gabbi, A. M., Silvio, R. O. & Costa Jr, J. B. G. (2016). Infrared thermography in animal production: An overview. Computers and Electronics in Agriculture, 123, 10-16. https://doi.org/10.1016/j.compag.2016.01.027
Molony, V., Kent, J. E., Viñuela-Fernández, I., Anderson, C., & Dwyer, C. M. (2012). Pain in lambs castrated at 2 days using novel smaller and tighter rubber rings without and with local anaesthetic. The Veterinary Journal, 193(1), 81-86. https://doi.org/10.1016/j.tvjl.2011.09.030
Niven, D. J., Gaudet, J. E., Laupland, K. B., Mrklas, K. J., Roberts, D. J., & Stelfox, H. T. (2015). Accuracy of peripheral thermometers for estimating temperature: a systematic review and meta-analysis. Annals of internal medicine, 163(10), 768-777. https://doi.org/10.7326/M15-1150
Ozaki, R., Inoue, S., Yorozui, Y., Ichikawa, R., Yamada, N., Higashi, S., Matsuyama, S., Tsukamura, H., Ohkura, S., Uenoyama, Y. & Morita, Y. (2024). Capturing temperature changes on the ocular surface along with estrus and ovulation using infrared thermography in Japanese Black cows. Journal of Reproduction and Development, 70(1), 49-54. https://doi.org/10.1262/jrd.2022-116
Pecoraro, V., Petri, D., Costantino, G., Squizzato, A., Moja, L., Virgili, G., & Lucenteforte, E. (2021). The diagnostic accuracy of digital, infrared and mercury-in-glass thermometers in measuring body temperature: a systematic review and network meta-analysis. Internal and emergency medicine, 16, 1071-1083. https://doi.org/10.1007/s11739-020-02556-0
Proctor, H., & Carder, G. (2016). Can changes in nasal temperature be used as an indicator of emotional state in cows?. Applied Animal Behaviour Science, 184, 1-6. https://doi.org/10.1016/j.applanim.2016.07.013
Soerensen, D. D., & Pedersen, L. J. (2015). Infrared skin temperature measurements for monitoring health in pigs: a review. Acta veterinaria scandinavica, 57, 1-11. https://doi.org/10.1186/s13028-015-0094-2
Sullivan, S. J., Rinaldi, J. E., Hariharan, P., Casamento, J. P., Baek, S., Seay, N., Vesnovsky, O. & Topoleski, L. T. (2021). Clinical evaluation of non-contact infrared thermometers. Scientific reports, 11(1), 22079. https://doi.org/10.1038/s41598-021-99300-1
Weaver, S. J., Hynd, P. I., Ralph, C. R., Edwards, J. H., Burnard, C. L., Narayan, E., & Tilbrook, A. J. (2021). Chronic elevation of plasma cortisol causes differential expression of predominating glucocorticoid in plasma, saliva, fecal, and wool matrices in sheep. Domestic Animal Endocrinology, 74, 106503. https://doi.org/10.1016/j.domaniend.2020.106503
Zebaria, H. M., Hidayet, H. M., Al-Naqshabendy, A. A., Hussein, N. J., & Kakarash, N. A. (2021). Pain caused by ear tagging in kids of native black goats. Science Journal of University of Zakho, 9(1), 15-19. DOI: https://doi.org/10.25271/sjuoz.2021.9.1.781
Authors
Copyright (c) 2025 Nizar J. Hussein, Rekesh S. Habib, Fatah M. Khalaf, Rana M. Fazal, Muhammad Owais

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License [CC BY-NC-SA 4.0] that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work, with an acknowledgment of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online.